Choosing the best conditions
When designing a process used in the manufacture of a chemical, a chemical engineer will need to decide on the conditions to use in that process. This will involve decisions regarding the temperature, the amount of reactants, the pressure, whether a catalyst is used and many other problems. If the process involves a reversible reaction, the aim will be to maximise the yield of the reaction.
It is also important to ensure that the rate of the reaction is fast enough. Otherwise a reaction that potentially has a high yield, may not be viable because it will take too long to make the product.
Often a compromise set of conditions needs to be used, to ensure the equilibrium yield and the rate of the reaction are high enough to make the process worthwhile.
Example
Look at the following process which shows the reaction of hydrogen and carbon dioxide to form the fuel methanol.
2 H2(g) + CO(g) 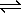 CH3OH(g) + heat
CH3OH(g) + heat
Below are a list of conditions which could affect the reaction.
Now look at your list of answers.
- Are there any conditions that increase the yield, but decrease the rate, or any that will increase the rate, but decrease the yield?
- What do you think the chemical engineer does when deciding the conditions used in this process?
Answer those questions for yourself and then click here for feedback.
Low temperature would increase the yield of the reaction, but high temperature is required to speed up the reaction. Therefore a compromise temperature will need to be chosen for this process. This will be high enough for the reaction to occur at a reasonable rate, but low enough to ensure an acceptable yield.
Research the actual conditions used for the industrial production of methanol to see how the problem can be addressed.

Access some problems  relating to situations where compromise sets of conditions are required.
relating to situations where compromise sets of conditions are required.

- Why do industrial plants try to avoid operating at very high pressures?
- Why are catalysts used extensively in industrial processes?





