Substitution reactions
The range of uses of hydrocarbons is fairly limited because they contain only carbon and hydrogen. This is why the main uses of hydrocarbons are as fuels. One of the ways that hydrocarbons can be converted to more interesting and useful products is through substitution reactions. The products are called substituted compounds and are formed when a hydrogen atom in the hydrocarbon is replaced (substituted) by another atom or group.
With these reactions methane can become chloromethane, benzene can become methyl benzene and propane can become 1-chloropropane.
The process for the reaction between propane and chlorine is shown in the video below.

Text alternative to the propane and chlorine video
The reaction between propane (CH3CH2CH3) and chlorine (Cl2) is shown in the form of an animation. The bond in the chlorine molecule breaks and one of the chlorine atoms replaces a hydrogen atom from the propane molecule to form 1-chloropropane (CH2ClCH2CH3).

Click here to open the video in a new window

This reaction requires a high level of energy to activate the reaction. Heat energy (infra-red radiation) is normally not strong enough to initiate the reaction, and ultraviolet (UV) radiation is required. Therefore, these types of reactions are often occurring in the upper atmosphere where the molecules are exposed to high levels of UV radiation from the sun.
The energy splits the reacting molecule. The particle produced by this splitting will add onto the hydrocarbon. Select the headings below to see the processes occurring in the above video.
In the above case the chlorine molecule can split apart when exposed to UV light as shown below.
Cl2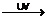 2 Cl∙
2 Cl∙

Chlorine atoms react with alkane
The single chlorine atoms can be represented as Cl∙ because they contain unpaired electrons on their outer shell as shown here.

Species with unpaired electrons are called radicals and are very reactive. In this case the chlorine radical  will react with the propane molecule as shown below.
will react with the propane molecule as shown below.
Cl∙ + CH3CH2CH3 → CH3CH2CH2Cl + H∙

The reactive hydrogen and chlorine radicals will rapidly pair up to form the more stable hydrogen chloride molecule.
H∙ + Cl∙ → HCl

Substitution reactions involve reactive radicals and other products are often formed as the radicals react with any species that happen to be close enough. The radicals rip electrons from any source in order to pair up the lone electron and complete their outer electron shell.
Predicting products from substitution reactions
It is relatively easy to predict the possible products from substitution reactions. The method involves the following steps.
- Split the reacting molecule into two parts.
- Remove a hydrogen atom from the hydrocarbon molecule.
- Replace the hydrogen atom with one of the parts of the reacting molecule.
This method can be illustrated by using molecular models if they are available.
The range of possible products is increased when the reacting molecule is present in excess. This is because the organic molecule might undergo more than one substitution reaction.
Complete the following questions to test your knowledge of substitution reactions.
1 |
Butane (CH3CH2CH2CH3) and iodine (I2)Which of the following could not be a product of this reaction if the substances were mixed together in the presence of UV light? |
2 |
Choosing reactantsWhich of the following reactants would be capable of producing 1,1-dibromoethane in a substitution reaction? |

Can you write a balanced equation for the reaction involved in the second question?
CH3CH3 + 2 HBr → CHBr2CH3 + 2 H2






